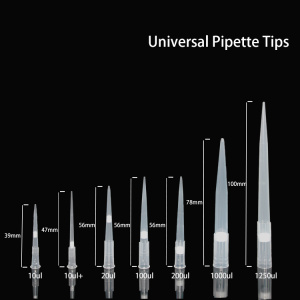
How pipettes are sterilized and sterilized
In the use of pipettes, the basic tool of molecular biotechnology, the concepts of disinfection and sterilization are often mixed. The difference between the two is that disinfection only requires that the live bacteria on the pipette be controlled within a certain range to achieve a harmless level, while sterilization requires the elimination of all live bacteria. Sterilization requires more processing than disinfection.
1. Chemical disinfection. Simply put, wipe the outer surface of the pipette with alcohol, etc., and then dry it. This should be possible for all pipette brands, otherwise the material of the housing is too bad!
2. UV disinfection. The surface of the pipette is irradiated with UV light, which sterilizes by destroying the DNA structure of cells. The duration of UV disinfection depends on the intensity of radiation and the resistance of bacteria to UV rays. Most brands of pipettes can be sterilized with UV light, but need to check with the supplier in advance.
3. The pipette is autoclaved. Generally speaking, the working conditions of high temperature and high pressure sterilization of pipettes are: 102.9kPa (1.05kg/cm2), 121℃, 20 minutes. The high temperature and high pressure sterilization of pipettes is divided into whole sterilization and half sterilization. It is absolutely possible that most brands of pipettes can be sterilized in half, but whether the whole pipe can be sterilized, the user must contact the supplier business confirmation. On the market, products such as manual pipettes can be sterilized as a whole. However, here are a few points to remind: Except for individual applications, such as infectious virus research, most customers do not need to sterilize the whole tube; second, for products of the same brand, the whole tube is often more expensive than the half tube sterilization. , customers need to make decisions according to their own needs.