Complete the nucleic acid test for all employees within 2 days within 5 million people! Let's take a look at Yongyue Medical's innovative solutions?
Some time ago
China's epidemic is occurring at multiple points and local outbreaks
Some areas have successively launched
Nucleic acid testing for all employees
Nucleic acid testing is a "monitoring network" for epidemic prevention and control
On August 10
China Health Commission issued
"Notice on Further Strengthening the Organization and Management of Nucleic Acid Testing for All Staff"
Requirements for the detection speed of different populations
The notice stated that in the event of an epidemic, an emergency response should be initiated immediately and the emergency command system should be activated. After confirming the implementation of nucleic acid testing for all employees, organize the implementation of the implementation plan for nucleic acid testing for all employees as soon as possible to ensure that the number of people tested within 5 million will be completed within 2 days, and those with more than 5 million tested will be completed within 3 days.
Since the outbreak
The demand for medical plastic consumables related to nucleic acid testing has increased
Injection molding and automated production equipment are also attracting attention
Nasopharyngeal swab that can be produced quickly
Every country is producing millions of nasopharyngeal swabs for COVID-19 testing. The only question is how fast can we start production?
Yongyue Medical used Allrounder and Multilift Select, and started mass production in just 14 days.

Nasopharyngeal swabs are currently entirely made of plastic raw materials through injection molding. The company currently produces 30,000 swabs around the clock. Arburg`s Allrounder 420C Gold Edition machine is equipped with Yongyue Medical`s 8-cavity mold.
Operators work in three shifts, packing every 1,000 swabs, and then sterilizing them at Yongyue Medical. These kits are then ready for use.

Use these swabs to get liquid from the nose or throat for testing. To this end, each molded part weighing only 0.66 grams is equipped with barbs on the upper part of the tip. After taking it out, separate the upper part of the test rod from the handle through the break point, and pack it in a small test tube. Then sent to the laboratory to analyze the data.
According to reports, from May to July, Yongyue Medical produced a total of 2.8 million pieces, which is equivalent to 30,000 pieces of products per day. And has applied for CE and FDA approval.

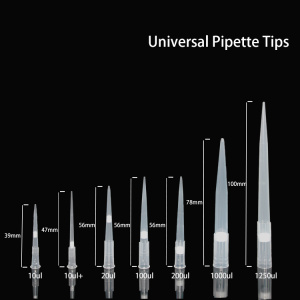
Automatic manufacturing system for pipette tips
Because of the epidemic, the in vitro diagnostics market has exploded geometrically this year, and the liquid handling methods required in the diagnosis are jointly completed by pipettes and pipettes.

Yongyue Medical`s automated production of pipette tips has good flexibility to adapt to pipette tips of different sizes. This solution is mainly produced by Yongyue Medical's 470H hydraulic press with a clamping force of 100 tons and three sets of automation modules.


Compared with most cold runners in China, where the number of cavities is only 16 or 32 cavities, the mold provided by Jiesta achieves 1 mold with 48 cavities. The single-point side injection hot runner technology ensures the multi-point injection Balance, and greatly reduce the pressure and cost of raw material supply, and eliminate the troubles of cold head processing. When the filling process reaches 30%, the material has been balanced to wrap the mold core, and the stable production quality has brought greater production capacity.

The material used in this product is black conductive PP, and the overall cycle time including the time in the manipulator mold is 6.9 seconds, and the fastest can reach 5.5 seconds.

COC material is used for nucleic acid detection microtiter plate
Yongyue Medical, a leading supplier of liquid transfer equipment for medical testing, has launched a polymerase chain reaction plate (for nucleic acid detection and other purposes) processed with Polyplastics TOPAS®COC material.
The TOPAS® COC material used in this SBS standard 96-well microtiter plate for genome testing has higher rigidity and thermal stability than polypropylene (PP) and polycarbonate (PC).

✔TOPAS®COC can meet the temperature cycle test of up to 100℃ in terms of thermal stability;
✔ Its high rigidity is convenient for the automated operation of the robot;
✔ Its excellent optical properties (92% light transmittance) help to improve the reliability of laser detection and real-time observation;
✔ The high fluidity of TOPAS® allows more details to be designed into PCR plates and other disposable diagnostic and microfluidic components;
✔ The number of holes can be maximized, channels can be merged and analysis can be optimized. Compared with competing materials, the ultra-high purity TOPAS® has extremely low extracts and extracts, so that the most reliable and reproducible test results can be obtained;
✔The inertness of medical-specific materials can prevent interference with detection reaction and analysis, and further improve accuracy;
✔ In addition, TOPAS also has important characteristics such as excellent ultraviolet transmission and low birefringence.